Disinfectant Compatibility Testing Clarifies Need For Better IFUs

by Barbara Strain, MA, SM(ASCP), CVAHP and Dr. Keri Lestage, Ph.D., PMP
Disinfectant labels provide a wealth of information about a product, including the microorganisms the product is effective against. However, how a disinfectant is used is critical to the efficacy of the product. One of the most important – but often overlooked – elements of a disinfectant label is the instructions for use (IFU). Healthcare providers must familiarize themselves with disinfectant IFUs to understand the product’s contact time, concentration, application method, and storage stability. If compliance with the disinfectant’s IFU fails, safe and effective disinfection may not occur.
Disinfectant Use on Medical Devices – Compatibility Issues
Now let’s consider the medical devices that these disinfectants are routinely applied to. Each medical device also has its own IFU with cleaning and disinfecting instructions. However, those instructions often don’t consider what disinfectants the facility has available or the equipment turn-over time. This puts healthcare facilities in a difficult position since misuse of disinfectants often leads to damaged equipment that takes them out of service, leading to delays in effective care. Healthcare facilities maintain statistics on the frequency of these occurrences since each event is logged into a reporting system by the biomedical department for further investigation and analysis.
In many cases, equipment damage or malfunction stems from disinfectant incompatibility or misuse. Failing to adhere to IFUs regarding concentration or contact time is often to blame. However, even strict adherence to IFUs may not prevent equipment damage due to disinfectant incompatibility. This fact should spark a call to action for healthcare facilities to share data on the number of incidents where equipment was pulled from service due to disinfectant incompatibility. Only when this problem’s extent is broadly shared will it compel action.
Disinfectant Manufacturers Are Starting to Address Material Compatibility
In the spirit of trying to address material compatibility issues in the field, many disinfectant and medical device manufacturers have developed dedicated internal resources that test formulations against commonly used plastics and metals. GAMA Healthcare is a good example of this. At the Infection Prevention Conference 2023, material science technologist Jake Jennings and the GAMA team presented their findings on material compatibility with thirteen plastics commonly used in medical devices. What made this study intriguing is that it didn’t test disinfectants at all. Although disinfectants have been shown to cause damage to many different types of surface materials, GAMA’s data showed that detergents can also be a major contributor to cracking and crazing of plastics.
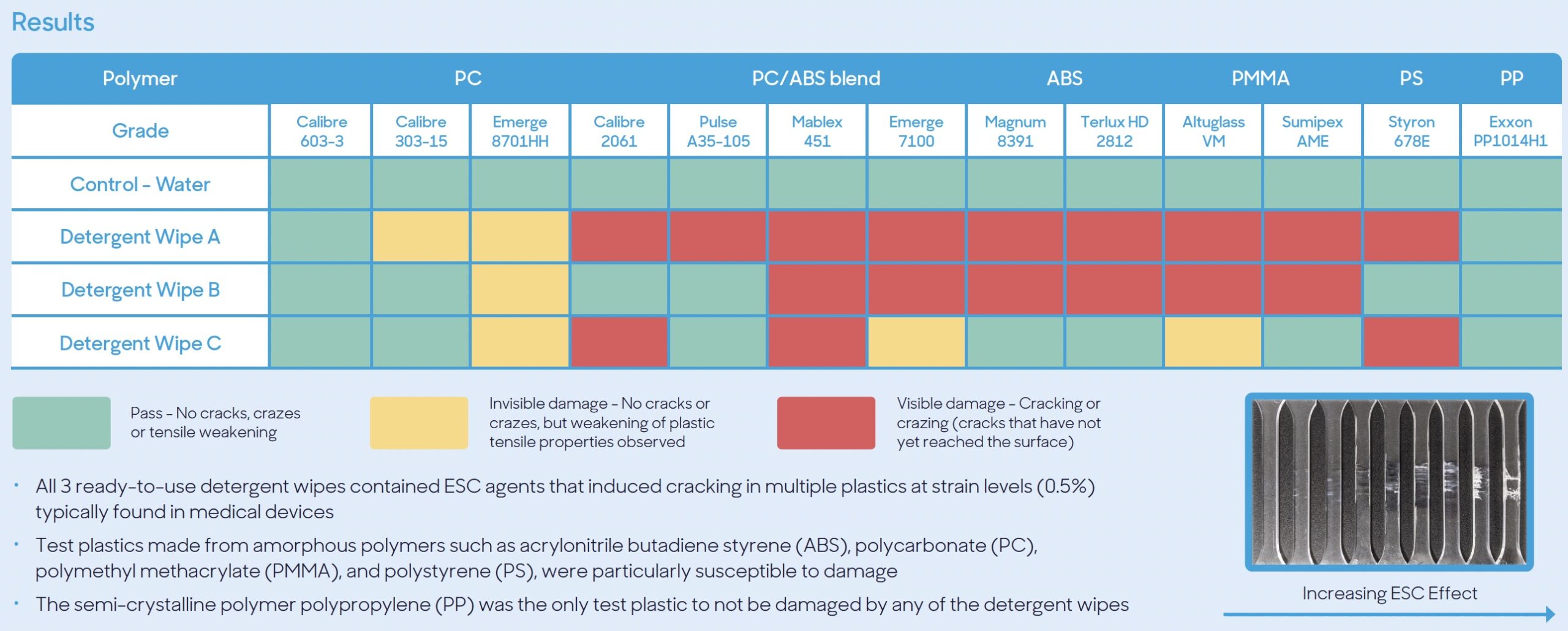
GAMA found that all 3 ready-to-use detergent wipes tested contained ESC agents that induced cracking in multiple plastics at strain levels (0.5%) typically found in medical devices. See the GAMA report here.
The growing understanding of compatibility issues underscores the importance of collaboration among disinfectant providers, medical device manufacturers, and healthcare facilities. While sharing knowledge among large, often competitive companies may be uncommon, collaborative alliances have proven to be incredibly powerful and lead to solutions addressing these complex challenges.
In a published example of successful collaboration, an HSI Case Study highlighted the importance of effective communication between organizations in swiftly resolving issues. In this instance, a hospital faced a significant material incompatibility problem that posed a risk to patient safety while also incurring substantial financial losses for both the hospital and the device manufacturer. However, once a direct line of communication was established between the hospital’s Value Analysis staff and the medical device manufacturer, both parties were able to expedite the development of a viable solution, effectively addressing the issue.
Collaboration is a fundamental principle of the Healthcare Surfaces Institute. The organization acts as a hub that facilitates identifying issues, sharing constraints, and streamlining efforts toward actionable solutions, but it starts with a willingness to participate.
Learn more about HSI’s volunteer, membership, and sponsorship opportunities. Together, we can make a difference.
As a 501(c)(3) non-profit organization, we rely on donations to save lives. We need your help.
Please consider donating, becoming a member, or exploring corporate sponsorship through your workplace today.

